Marker Formation in Synthetic Methyl Salicylate
May 10, 2024 📙 2 min read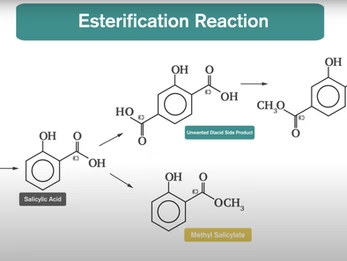
Wintergreen and birchwood essential oils contain more than 98% methyl salicylate. As high-priced essential oils, they are prone to adulteration with synthetic methyl salicylate derived from phenol (a fossil fuel-based precursor). Any addition of synthetic methyl salicylate to wintergreen or birchwood oil would not change the percentages of minor components significantly—so monitoring minor components can fail in this case. Chiral ratios can authenticate oils containing chiral molecules, but methyl salicylate is non-chiral, so chiral GC–MS will not help here.
Synthetic methyl salicylate is derived from the petrochemical-based precursor phenol, so C14 testing can be useful for detecting this type of adulteration. APRC has also observed that C14 testing can have limitations for identifying synthetic methyl salicylate, with accuracy varying by about 3–5% when detecting synthetic methyl salicylate by C14 testing.
APRC has found that a synthetic marker-based technique can be more reliable for certain adulteration scenarios. In the synthetic process of methyl salicylate from phenol, a trace byproduct called dimethyl 2-hydroxyterephthalate can form, which can serve as a synthetic marker for methyl salicylate. If a lab is able to detect these markers properly, it may be possible to identify very small amounts of synthetic methyl salicylate—even at additions below 1%—in wintergreen or birchwood essential oils.
Watch the synthetic route and mechanism of marker formation in synthetic methyl salicylate: https://youtu.be/za79Kaf9Szo





