Osmanthus Adulteration
May 10, 2024 📙 2 min read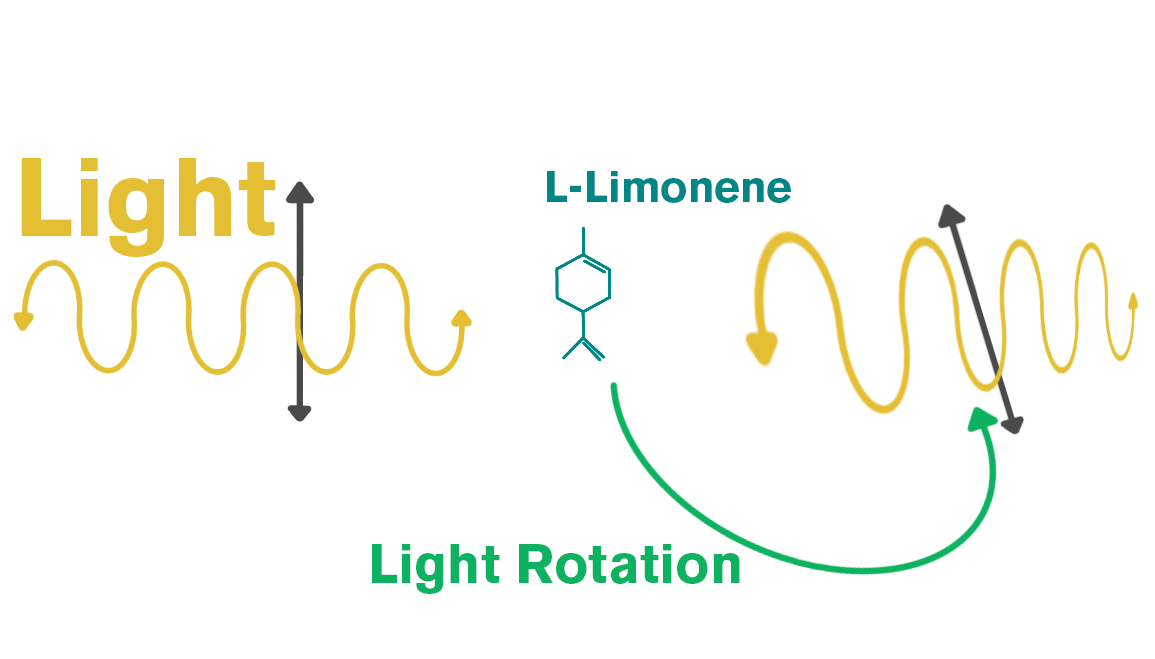
Osmanthus absolute is a rare and expensive product on the market. This is partially due to the compound ionone, which is a perfume-quality molecule. Due to the price, osmanthus absolute is often adulterated. This absolute is usually adulterated with a carrier or cooking oil; however, most of the compounds in osmanthus are commercially available, so synthetic compounds may also be added. Fractionated geraniol from palmarosa and linalool from ho wood are also readily attainable. Linalool oxide (cis and trans) can be produced from oxidized linalool.
Due to the ease of adulterating this absolute, it’s essential to have a lab test it for purity. One interesting fact about osmanthus is that it contains several chiral molecules. Essential oils can have chiral and non-chiral molecules. A molecule is chiral when light passes through one form in a clockwise direction and through another form in a counter-clockwise direction. For example, limonene is chiral: if light passes through it clockwise, it’s D-(+)-limonene; if counterclockwise, it’s L-(-)-limonene.
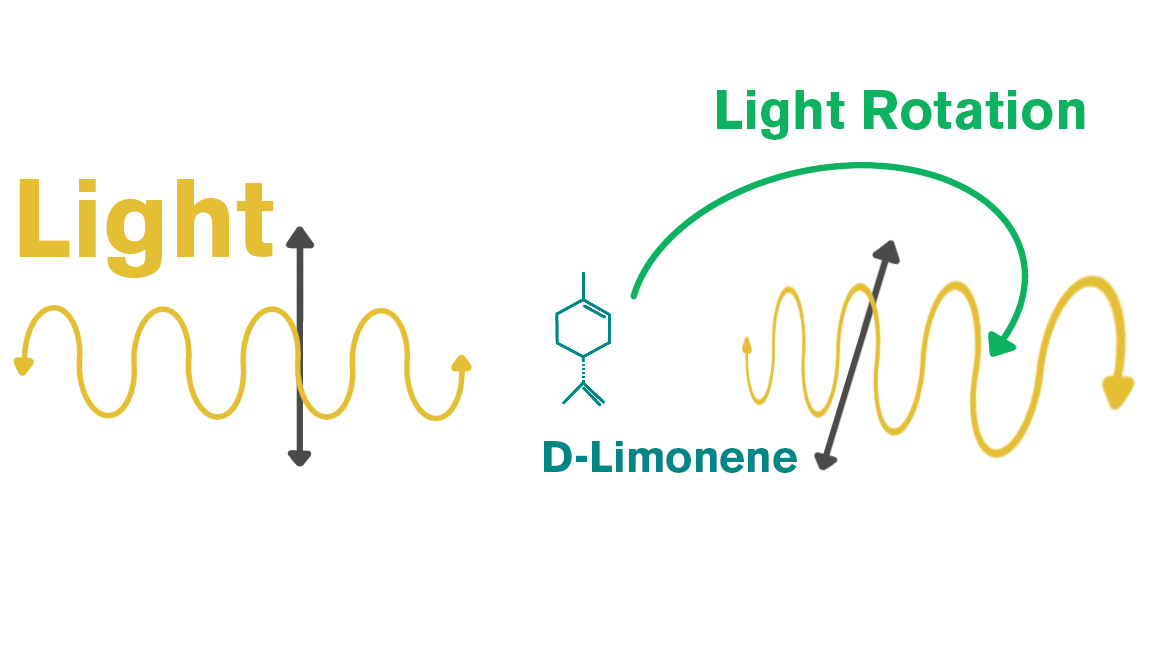
Essential oils can contain both D and L forms. A standard GC–MS can identify total compounds in an oil, but chiral GC can determine the percentage of each enantiomer. For example, an oil may have 6% total linalool, while chiral GC can show that 80% of that linalool is L-linalool and 20% is D-linalool.
Chirality is typically consistent in an oil within a small range. So an oil might have 6–15% linalool, but it may consistently show ~79–81% L-linalool and ~19–21% D-linalool. If a sample shows enantiomeric ratios far outside those expected ranges, it can indicate adulteration. APRC offers chiral GC testing because chirality is a powerful tool for confirming authenticity.





